BY LETTER
Nucleosynthesis
Nucleosynthesis is the process that produces new elements, that occurs naturally in high energy environments, and also as a result of artificial manufacturing processes. The first three minutes of the Big Bang was a very hot, dense, energetic environment, and nucleosynthesis occurred in this brief period, producing almost all the hydrogen now present in the universe as well as significant amounts of helium and much smaller amounts of lithium and boron.
Slow Stellar Nucleosynthesis
After the Big Bang and the inflationary period, Stellar Nucleosynthesis started to occur in the first stars, producing much more helium via various hydrogen-based fusion processes. As the stars got larger and older, heavier elements were produced via different types of helium fusion, leading to the creation of carbon and nitrogen in particular.A range of elements with higher atomic numbers are produced via the slow, 's-process' nucleosynthesis that occurs in the asymptotic red giant phase. These dying old stars emit these heavier elements in their stellar winds as they die, especially during energetic events known as 'dredge-ups'; these winds and events enrich the interstellar medium. Elements produced by dying low-mass stars include nitrogen and carbon (and some lithium), as well as a number of elements heavier than iron such as barium, mercury, lead and tungsten (see the periodic table below for more details).
Although dying low-mass stars also produce oxygen, this element usually remains near the core and is not released in the red giant phase, and generally ends up in the stellar remnant (a white dwarf).
Fast (Explosive) Nucleosynthesis
Note that fusion reactions up to iron are exothermic, but endothermic reactions which produce heavier elements can occur via both fast and slow synthesis processes. Many elements with higher atomic mass are produced in rapid (r-process) nucleosynthesis associated with very high energy events, such as exploding white dwarf stars, core-collapse supernovae and colliding neutron stars. These rapid events can produce very large amounts of heavy elements in a very short period, sometimes a few minutes; this is in sharp contrast with s-process nucleosynthesis that occurs over millions of years.Exploding white dwarf stars (novas) produce significant amounts of calcium, iron, nickel, sulphur, potassium, copper and zinc. White dwarfs consist mainly of carbon or oxygen; these elements mostly remain in their interior regions, although they can be released during class 1a supernovae events, which are relatively infrequent.
Inside very massive stars, carbon, neon, oxygen, silicon and finally iron are produced in concentric shells. These elements are ejected during core-collapse supernovae events, which also produce many other elements very rapidly during the catastrophic explosion via r-process nucleosynthesis. These elements include sodium, magnesium, potassium, aluminium, calcium, as well as several heavier elements such as nickel, copper, zinc, selenium, krypton and germanium.
 Image from Steve Bowers | |
| Colliding neutron stars produce about half of all the isotopes heavier than iron in the interstellar medium | |
A number of other nucleosynthesis processes occur in various environments, such as the p-process, rp-process and cosmic ray fusion (this last process is responsible for the production of boron and beryllium, and some lithium, via cosmic ray spallation events on or near the surface of planetary objects).
Note as well that nucleosynthesis processes often produce short-lived radioactive elements and isotopes which quickly decay into other, more stable elements. For more details about the relative cosmic abundance of elements, see this page.
Black hole accretion discs, especially discs which extract material from a binary stellar partner, are also seats of nucleosynthesis, although their contribution to enrichment of the interstellar medium is minimal. This process can however be utilised in artificial nucleosynthesis projects using tailor-made black holes.
Artificial nucleosynthesis
Artificial nucleosynthesis was first achieved on Old Earth in the so-called Atomic Age; since that time the artificial production of elements from abundant hydrogen and helium has become relatively commonplace. The practice of elemental synthesis is referred to as alchemics.Large scale transmutation is a high-energy activity, often using massive reactors such as DWIZ black hole accretion disks that can replicate many s-process and r-process forms of synthesis, but these systems operate at very high-temperatures and emit many forms of dangerous radiation, particularly neutrons. A DWIZ is often created in orbit around a large gas giant or brown dwarf, where large quantities of matter (especially hydrogen and helium) can be introduced into the zone in a controlled manner, promoting a wide variety of fusion reactions.
Fusion reactions also occur in power production and spacecraft propulsion and produce a number of elements as waste products, but small-scale nucleosynthesis to produce limited quantities of more useful elements requires extremely advanced technology at the ultratech level and beyond, and is generally used for specialist projects only.
Related Articles
- Alchemics
- Black Hole
- Deep Well Industrial Zone
- Interstellar Medium
- Neutron Star
- Nuclear Engineering
- Nuclear Fusion
- Nuclear Reactor - Text by M. Alan Kazlev
A power plant that uses controlled atomic fission or fusion to generate energy. - Nucleosynthetic Era - Text by M. Alan Kazlev
The era following the Leptonic Era, between 1 second and 1,000 seconds after the Big Bang, in which light elements (helium and deuterium) are synthesized during the hot early phases of the hot Big Bang. - Nucleus, Atomic - Text by M. Alan Kazlev
The central part of an atom, made up of protons and neutrons, and containing nearly all of the atomic mass. - Relative Cosmic Abundance of Elements
- Supernova
- Supernova Remnant
Appears in Topics
Development Notes
Text by Steve Bowers
From an original short article by M. Alan Kazlev
Initially published on 09 December 2001.
From an original short article by M. Alan Kazlev
Initially published on 09 December 2001.
Additional Information
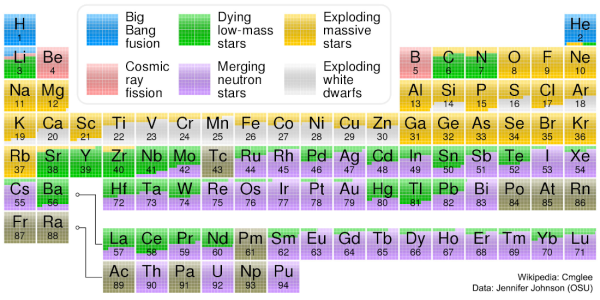
The origin of the chemical elements Chart prepared by Cmglee based on work by Jennifer Johnson at Ohio State University. Used under Creative Commons Attribution-Share Alike 3.0 Unported licence.
------------------
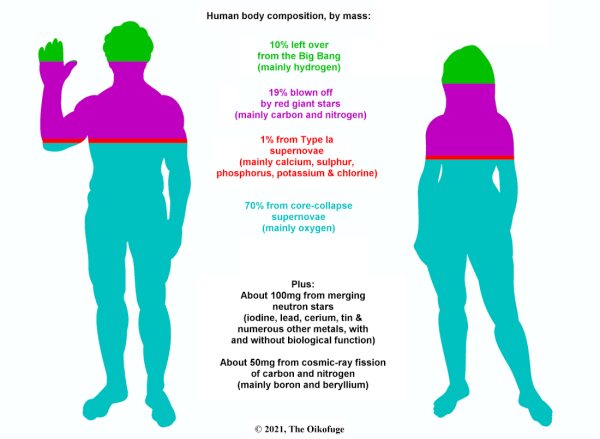
The image 'Composition of the Human Body by Mass' by Grant Hutchison, image copyright and used with permission. Read more about this image here
------------------
The image 'Composition of the Human Body by Mass' by Grant Hutchison, image copyright and used with permission. Read more about this image here