BY LETTER
Barocapnic Life
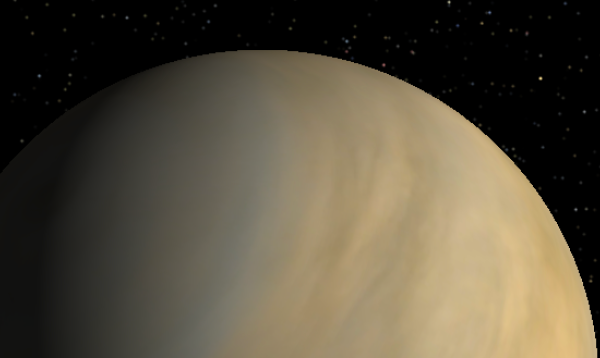 Image from Steve Bowers | |
| Scaramouche, a Capnocytherian world with Barocapnic life | |
At such temperatures, carbon based polymers and single-chain silicone polymers decompose, so barocapnic life tends to rely on double-chain silicones and/or silicate-like polymer backbones.
Barocapnic life faces two obstacles which make it rarer than aqueous-carbon life.
First, silicates are poorly soluble in supercritical carbon dioxide, which tends to inhibit complex life unless it uses a secondary solvent such as molten salt.
Second, EuCytherean worlds have a low abundance of hydrogen. Emerging barocapnic life can sometimes create a "cloudless catastrophe" which renders it extinct. If the local biosphere sequesters hydrogen, it can prevent the formation of sulphuric acid clouds in the upper atmosphere. Consequently, the planet absorbs more sunlight and its temperature increases, destroying the biosphere.
Notable worlds with barocapnic life include:
Lin Daiyu, where the first example of simple barocapnic life was discovered.
Reshlak, where the first example of complex barocapnic life was discovered.
Scaramouche, home of the only known barocapnic xenosophonts, the Riposte.
Related Articles
Appears in Topics
Development Notes
Text by Liam Jones
Initially published on 04 August 2024.
Initially published on 04 August 2024.