Posts: 1,690
Threads: 261
Joined: Apr 2013
If you had a mostly-nitrogen/CO2 atmosphere (~80 / 20%) with some hydrocarbons (e.g., methane) in it, what percentage of hydrocarbon in the atmosphere would be required to support combustion if an oxidizer were supplied? Is 2-3% enough, 5%, or over 10% necessary?
Mike Miller, Materials Engineer
----------------------
"Everbody's always in favor of saving Hitler's brain, but when you put it in the body of a great white shark, oh, suddenly you've gone too far." -- Professor Farnsworth, Futurama
Posts: 11,715
Threads: 453
Joined: Apr 2013
I don't know, although there are a couple of forums which I frequent where the posters might have a better idea.
I suspect the methane level would need to exceed 10%, since it is supporting combustion like oxygen does in our atmosphere. If the O2 level in air decreases much below 10% then fire becomes problematic, although this is probably also influenced by partial pressure.
Posts: 11,715
Threads: 453
Joined: Apr 2013
This question doesn't seem to have been asked very often in the past, so I'm not finding many people who are willing to come forward as an expert on the matter.
Best answer so far
http://cosmoquest.org/forum/showthread.p...ost2261512
kzb Wrote:I don't know for a fact.
However that won't stop me. The flammability limit for methane in air is 4.4 to 15%.
Perhaps to a reasonable approximation it is symmetrical, so if you had as little as 4.4% methane in the atmosphere then pure oxygen would burn in that.
Bear in mind that CH4 + 202 = CO2 + 2H20
There are two volumes of oxygen per unit volume of methane, so perhaps it is reasonable to expect a lower percentage of methane supporting an oxygen flame than the other way round.
Posts: 11,715
Threads: 453
Joined: Apr 2013
12-19-2014, 05:03 AM
(This post was last modified: 12-19-2014, 05:04 AM by stevebowers.)
Googling around I found this thread about Titan on a forum I'm not a member of;
http://www.unmannedspaceflight.com/index...topic=2833
Quote:The surface temperature of titan is actually well above the flash point of methane (-178C vs. -223C). Furthermore, we know now that at the surface, the concentration of methane gas is ~5%, this is within the explosion fraction range of methane of 5-15% on earth with an O2 conc. of 20% (the fact that we are dealing with gas at the surface actually negates the relevancy of methane's flash point). I also suspect that because the pressure of the atmosphere at the surface is higher than at earth's surface, the explosion fraction for methane would actually slightly extend below the 5% lower limit add to this the fact that you'd be burning it with 100% O2 instead of earth's paltry 20% and this probably pushes the explosion fraction limits even wider. The fact that the gas is very cold is irrelevant. What's -180C when you're talking about a flame temperature in the thousands of degrees? nothing. It is quite certain that you could light a flame off of a bottle of O2 at the surface and it would self sustain 'till it ran out. Even if you are uncomfortable with the closeness to the lower limit of the explosion fraction at 5%, performing a concentration of methane out of the atmosphere to a slightly higher % would be trivially easy with a semipermiable membrane and a very small amount of energy input (the amount of entropy change you'd need to concentrate it to say, 10% would be very small). The nice thing about the ability to carry liquid O2 instead of liquid hydrocarbon is the high density. a small bottle of O2 would go a long way. Even if you wanted to run some silly scheme of condensing the methane out of the atmosphere as a liquid using a cryocooling loop and then heating and burning off the purified lquid all at once (like for a rocket), this would be VERY easy and energetically inexpensive to do since you're already so close to the boiling point.
Posts: 11,715
Threads: 453
Joined: Apr 2013
Another addition to the data has now been added by Grant Hutchison;
http://cosmoquest.org/forum/showthread.p...ost2261587
Quote:you need a flammability diagram for methane. The gases have to be present in the volume ratios shown, so atmospheric pressure is irrelevant except that higher pressure mixtures will produce bigger bangs or hotter flames.
In your example of an oxygen jet, combustion can take place at the edges of the jet where the oxygen has turbulently mixed with the atmosphere to produce a mix in the combustible range. New combustible mix forms constantly at the boundaries of the jet, and is heated by the combustion process until it combusts, too. The combustion can't spread into the jet or out into the atmosphere, because the mix shifts outside the combustible zone. So you produce a self-sustaining flame which is essentially an inside-out version of a methane jet burning in air.
![[Image: methane_flammability_diagram_sr.jpg]](http://cfbt-us.com/wordpress/wp-content/uploads/2009/04/methane_flammability_diagram_sr.jpg)
I love triangular graphs like this.
Posts: 11,715
Threads: 453
Joined: Apr 2013
On reading a ternary diagram, Grant has this to say;
Quote:Drawing a line from the 100% oxygen corner to the passive agent axis gives you the gas mixes possible around a pure oxygen jet in the atmospheric mix of your choice. If the atmosphere is less than 7% methane (more than 93% inert), the line never intersects the combustion zone, so that's the cut-off you were looking for in the OP. Slightly more than 7% methane will allow combustion, but it might be so marginal it was difficult to sustain as a flame.
An atmosphere of 11% methane (89% inert) crosses the stoichiometric point for air/methane mixtures, so a pure oxygen jet in that atmosphere should produce combustion similar to burning a pure methane jet in air.
And more than 11% methane in your atmosphere and you're going to get good sustained combustion friom your scenario, unless you have very low pressures in your mix.
note that Titan only has a 1.4% methane content in its atmosphere, according to Wiki, so no flames there.
Posts: 1,690
Threads: 261
Joined: Apr 2013
Dude, have a brownie point. Even a "special" brownie point from Colorado. That was some solid research and crunchy numbers.
Stay tune for atmospheric questions on my Mega-Mars thread.

Mike Miller, Materials Engineer
----------------------
"Everbody's always in favor of saving Hitler's brain, but when you put it in the body of a great white shark, oh, suddenly you've gone too far." -- Professor Farnsworth, Futurama


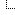


![[Image: methane_flammability_diagram_sr.jpg]](http://cfbt-us.com/wordpress/wp-content/uploads/2009/04/methane_flammability_diagram_sr.jpg)
