BY LETTER
Helium
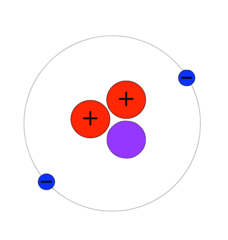
The second lightest naturally occurring atomic element, and the most common in the universe after hydrogen. About one quarter of all conventional matter in the universe is helium, and it is plentiful in stars, the atmosphere of gas giants, and the interstellar gaseous medium, though rare on smaller rocky or icy planets and moons lacking the gravity to retain it. Aside from the quantities formed in the original nucleosynthesis of the Big Bang, helium is also created from hydrogen atoms in the process of nuclear fusion that occurs within stars. The most common isotope of helium has two protons, two neutrons, and two electrons.
Rarer isotopes of helium are important in fusion reactor technology. Helium-3 (He-3, also written as 3He) is a light, non-radioactive isotope of helium with two protons and one neutron, in contrast with two neutrons in common helium. Helium-3 was a valuable fuel before the development of conversion reactors, and is still used in conversion-fusion reactors, because of its low neutron emission characteristics.
Helium is the lightest noble gas, and because it is chemically inert it is very popular as the lifting gas in dirigibles, blimps and airships on worlds or on large habs with a standard Terragen atmosphere.
Rarer isotopes of helium are important in fusion reactor technology. Helium-3 (He-3, also written as 3He) is a light, non-radioactive isotope of helium with two protons and one neutron, in contrast with two neutrons in common helium. Helium-3 was a valuable fuel before the development of conversion reactors, and is still used in conversion-fusion reactors, because of its low neutron emission characteristics.
Helium is the lightest noble gas, and because it is chemically inert it is very popular as the lifting gas in dirigibles, blimps and airships on worlds or on large habs with a standard Terragen atmosphere.
Related Articles
- Adams Helium
- Helium Flash - Text by M. Alan Kazlev
Runaway and explosive helium fusion inside a star as it evolves off the main sequence and into the giant phase of evolution. It occurs when degenerate gas at the star's center reaches a temperature of about 108 K. It can be prevented, but this is costly and requires extensive stellar engineering - Relative Cosmic Abundance of Elements
Appears in Topics
Development Notes
Text by Stephen Inniss after the original by M. Alan Kazlev
Initially published on 07 November 2001.
Initially published on 07 November 2001.